Interview with Robert Greenberg, M.D., Ph.D., President of Second Sight, Sylmar, California
Share:
Beck: Hi Dr. Greenberg. It is so exciting to meet you and to tour your facility. Greenberg:Hi. Dr. Beck. It's a pleasure to have you here.Beck: Let's start with a little biographical information about you, so the readers will know who you are, and then we'll get to the very exciting work you're doin
Beck: Hi Dr. Greenberg. It is so exciting to meet you and to tour your facility.
Greenberg:Hi. Dr. Beck. It's a pleasure to have you here.
Beck: Let's start with a little biographical information about you, so the readers will know who you are, and then we'll get to the very exciting work you're doing here.
Greenberg:Sure. I went to Johns Hopkins and did my M.D. and Ph.D. graduate training there.
I was originally involved with electrical engineering. I finished my Ph.D in biomedical engineering and graduated from medical school in 1998. After completing my education, I went to work for the FDA, evaluating medical devices. My doctoral dissertation related to the theoretical analysis of electrical stimulation of the retina. Soon after that, early in 1999, I was asked to join Second Sight, and I thought it offered an amazing challenge and opportunity, and here I am!
Beck: And can you please tell me a little about the origin of Second Sight?
Greenberg: Sure. Second Sight was incorporated in 1998 and was founded by Alfred Mann, the founder of Advanced Bionics -- one of the leading companies in cochlear implants.
Beck: I had the honor of meeting and interviewing Dr. Mann a few years ago through Advanced Bionics. I suspect much of the foundation upon which the Second Sight technology is based, has to do with cochlear implant technology developed through Advanced Bionics?
Greenberg: Yes, the technology, research and collaboration with Advanced Bionics helped immensely with our Model 1 implant.
Beck: Would you please tell me a brief overview as to what you're doing and creating in these labs?
Greenberg: Our mission at Second Sight is to restore vision in patients blinded by outer retinal degenerations, such as; macular degeneration and retinitis pigmentosa (RP). There are some 3 million people with macular degeneration and about 300 thousand with RP in the western world, with more or less intact optic nerves and severe retinal damage. The RP people tend to go completely blind, and people with macular degeneration tend to lose their central vision while maintaining their peripheral vision, although there are exceptions.
Beck: Can you please describe the Second Sight device for me?
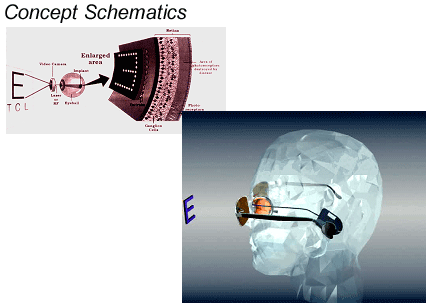
Greenberg: Sure, much like cochlear implants, we're going through development and generational changes. But the first device was based on the Clarion cochlear implant. We modified the electronics a bit, and then designed an entirely new flat, two dimensional electrode array, designed to sit on the retina, rather than within the cochlea. The first unit had 16 electrodes, and we had to design a tool the width of a human hair, to secure the device to the back of the eye. In the first photo, you can see the electrode as it would be placed on the retina. If you look carefully you can even see the 16 electrodes.
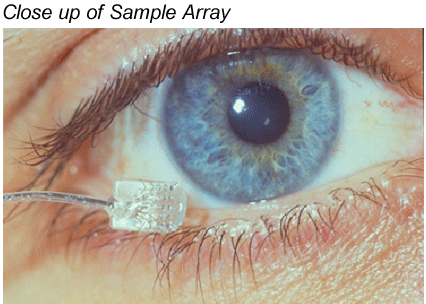
Beck: That is very impressive. So you essentially have a non-functioning end organ, in other words, the retina is not working, and then you have an intact optic nerve, and the electrode sends an electrical signal to the nerve cells within the retina and the optic nerve, and you get a visual image?
Greenberg: Yes, we're stimulating nerve cells within the retina, which then stimulate the optic nerve. So in that respect, it's the same as the cochlear implant.
Beck: And the unit receives its signal from what appears to be a camera mounted within a set of eyeglass frames. And the implanted electronics are located in the mastoid? And why did you start with 16 electrodes?
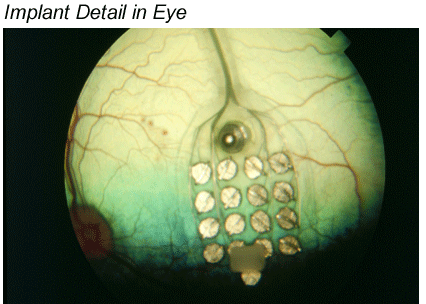
Greenberg: The 16 electrodes was the starting point because that's what was available through Advanced Bionics and because we were able to leverage the Advanced Bionics technology, and we soon became the leaders in the field. That was the first generation, we have developed it quite a bit past this, but here's another illustration, which helps explain what you just described. You can see in the photo below that the "communication point" between the implanted and the external equipment is about the same location as the implanted components of a cochlear implant. Of course there are differences as well as similarities between the two systems. For example, the retina is mapped in two dimensions, whereas the cochlea is mapped in one.
Beck: And both senses have end-organs that are topographically oriented too.
Greenberg: Sure. However, another difference is the cochlea is a very high frequency system, it works rapidly, and responds to the tiniest temporal cues. The visual system is much slower, and miniscule timing events are of less consequence with respect to vision.
Beck:What is the "target" with respect to the number of electrodes?
Greenberg:Excellent question. We started with 16 for the reasons noted above, and we originally thought 1000 was the goal. In other words, 1000 pixels really looked quite good to our "normal" eyes, so that became the theoretical target for a while....but frankly, 16 looks very good to our blind patients, and maybe the best number will turn out to be 256. It's a little too early to really know the answer, but the current target is in the hundreds. The patient with 16 electrodes can move their heads around a little, which enhances the image. They can identify large letters and they can tell the difference between a cup, a plate and a spoon...so it's pretty crude vision at the moment, but I believe it's going to improve rapidly. And even this crude vision appears helpful to completely blind people.
Beck: I suppose "neural plasticity" represents a major opportunity for your device?
Greenberg:Yes, it certainly does. Currently, none of our patients have taken the device home. They have the implant, but they just wear the external device here in the lab. So we don't really know how much adaptation will occur, or how quickly.
Beck: I am surprised to hear that. I assumed they were wearing the device at home, much like our cochlear implant patients did in the early days before the FDA approval? In fact, if cochlear implant patients had only worn the devices in the lab -- we might not have gotten out of the lab...as it can easily take a few days, weeks or months of daily use to really obtain good sound quality, and to recognize the sounds as meaningful words, music, and parts of speech.
Greenberg:I agree, that's an important milestone and we're just about to start letting them take the external unit home.
Beck: So this will get even more exciting as the subjects get more experience with the device. I suspect the goals and expectations will change as the device improves?
Greenberg:Yes, in fact, in some respects they change all the time. At first the goal was an awareness of light versus dark, or figure versus ground, and then discriminating between same versus different...but in the future, maybe one day it'll be watching CNN or driving a car! I know one common desire the patients express is seeing their grandchildren! We haven't yet really discovered the outer limits, so it's hard to define reasonable expectations...other than to be very cautious and very conservative as we step towards our goal of restoring sight to the blind.
Beck:I agree, you really want to under-promise and over-deliver as best you can. That too, is analogous to cochlear implants. In the early days we talked about the primary benefit being environmental sound awareness, and at this point, the vast majority of newly implanted people terrifically exceed that, and they can chat on the phone, and participate fully in most conversations...so "reasonable expectations" have evolved over time! What is the current FDA status?
Greenberg: We have two FDA clinical trials underway...One is a long term clinical trial with 6 patients, and then we have a short term study underway too, and that one is evaluating the next generation device. We're hoping to do a multi-center trial in the next year or two. As of this moment, the Doheny Eye Institute at USC is the only center working with us, and several of the scientists and physicians that worked with me at Johns Hopkins were recruited by Doheny, and they are among the best retinal surgeons and scientists in this field.
Beck:Dr. Greenberg, my recollection was that the first visual implant was done in concert with three surgeons...an ENT surgeon, a retinal surgeon and an oculoplastic surgeon?
Greenberg: Yes, that's right it was a team of surgeons. But for the second generation, it'll just be the retinal surgeon and all of the electronics will be located within the eye itself.
Beck: Things are changing very quickly for visual implants and Second Sight. This is very exciting, thanks for allowing me to visit with you, and I am so grateful for your time.
Greenberg: Thanks Dr. Beck. It's wonderful to share our progress with you, and I'm grateful for your continued interest.
- - - - - - -
For More information about Second Sight, CLICK HERE.
CLICK HERE for more information on Advanced Bionics.
Greenberg:Hi. Dr. Beck. It's a pleasure to have you here.
Beck: Let's start with a little biographical information about you, so the readers will know who you are, and then we'll get to the very exciting work you're doing here.
Greenberg:Sure. I went to Johns Hopkins and did my M.D. and Ph.D. graduate training there.
I was originally involved with electrical engineering. I finished my Ph.D in biomedical engineering and graduated from medical school in 1998. After completing my education, I went to work for the FDA, evaluating medical devices. My doctoral dissertation related to the theoretical analysis of electrical stimulation of the retina. Soon after that, early in 1999, I was asked to join Second Sight, and I thought it offered an amazing challenge and opportunity, and here I am!
Beck: And can you please tell me a little about the origin of Second Sight?
Greenberg: Sure. Second Sight was incorporated in 1998 and was founded by Alfred Mann, the founder of Advanced Bionics -- one of the leading companies in cochlear implants.
Beck: I had the honor of meeting and interviewing Dr. Mann a few years ago through Advanced Bionics. I suspect much of the foundation upon which the Second Sight technology is based, has to do with cochlear implant technology developed through Advanced Bionics?
Greenberg: Yes, the technology, research and collaboration with Advanced Bionics helped immensely with our Model 1 implant.
Beck: Would you please tell me a brief overview as to what you're doing and creating in these labs?
Greenberg: Our mission at Second Sight is to restore vision in patients blinded by outer retinal degenerations, such as; macular degeneration and retinitis pigmentosa (RP). There are some 3 million people with macular degeneration and about 300 thousand with RP in the western world, with more or less intact optic nerves and severe retinal damage. The RP people tend to go completely blind, and people with macular degeneration tend to lose their central vision while maintaining their peripheral vision, although there are exceptions.
Beck: Can you please describe the Second Sight device for me?
Greenberg: Sure, much like cochlear implants, we're going through development and generational changes. But the first device was based on the Clarion cochlear implant. We modified the electronics a bit, and then designed an entirely new flat, two dimensional electrode array, designed to sit on the retina, rather than within the cochlea. The first unit had 16 electrodes, and we had to design a tool the width of a human hair, to secure the device to the back of the eye. In the first photo, you can see the electrode as it would be placed on the retina. If you look carefully you can even see the 16 electrodes.


Beck: That is very impressive. So you essentially have a non-functioning end organ, in other words, the retina is not working, and then you have an intact optic nerve, and the electrode sends an electrical signal to the nerve cells within the retina and the optic nerve, and you get a visual image?
Greenberg: Yes, we're stimulating nerve cells within the retina, which then stimulate the optic nerve. So in that respect, it's the same as the cochlear implant.
Beck: And the unit receives its signal from what appears to be a camera mounted within a set of eyeglass frames. And the implanted electronics are located in the mastoid? And why did you start with 16 electrodes?
Greenberg: The 16 electrodes was the starting point because that's what was available through Advanced Bionics and because we were able to leverage the Advanced Bionics technology, and we soon became the leaders in the field. That was the first generation, we have developed it quite a bit past this, but here's another illustration, which helps explain what you just described. You can see in the photo below that the "communication point" between the implanted and the external equipment is about the same location as the implanted components of a cochlear implant. Of course there are differences as well as similarities between the two systems. For example, the retina is mapped in two dimensions, whereas the cochlea is mapped in one.

Beck: And both senses have end-organs that are topographically oriented too.
Greenberg: Sure. However, another difference is the cochlea is a very high frequency system, it works rapidly, and responds to the tiniest temporal cues. The visual system is much slower, and miniscule timing events are of less consequence with respect to vision.
Beck:What is the "target" with respect to the number of electrodes?
Greenberg:Excellent question. We started with 16 for the reasons noted above, and we originally thought 1000 was the goal. In other words, 1000 pixels really looked quite good to our "normal" eyes, so that became the theoretical target for a while....but frankly, 16 looks very good to our blind patients, and maybe the best number will turn out to be 256. It's a little too early to really know the answer, but the current target is in the hundreds. The patient with 16 electrodes can move their heads around a little, which enhances the image. They can identify large letters and they can tell the difference between a cup, a plate and a spoon...so it's pretty crude vision at the moment, but I believe it's going to improve rapidly. And even this crude vision appears helpful to completely blind people.
Beck: I suppose "neural plasticity" represents a major opportunity for your device?
Greenberg:Yes, it certainly does. Currently, none of our patients have taken the device home. They have the implant, but they just wear the external device here in the lab. So we don't really know how much adaptation will occur, or how quickly.
Beck: I am surprised to hear that. I assumed they were wearing the device at home, much like our cochlear implant patients did in the early days before the FDA approval? In fact, if cochlear implant patients had only worn the devices in the lab -- we might not have gotten out of the lab...as it can easily take a few days, weeks or months of daily use to really obtain good sound quality, and to recognize the sounds as meaningful words, music, and parts of speech.
Greenberg:I agree, that's an important milestone and we're just about to start letting them take the external unit home.
Beck: So this will get even more exciting as the subjects get more experience with the device. I suspect the goals and expectations will change as the device improves?
Greenberg:Yes, in fact, in some respects they change all the time. At first the goal was an awareness of light versus dark, or figure versus ground, and then discriminating between same versus different...but in the future, maybe one day it'll be watching CNN or driving a car! I know one common desire the patients express is seeing their grandchildren! We haven't yet really discovered the outer limits, so it's hard to define reasonable expectations...other than to be very cautious and very conservative as we step towards our goal of restoring sight to the blind.
Beck:I agree, you really want to under-promise and over-deliver as best you can. That too, is analogous to cochlear implants. In the early days we talked about the primary benefit being environmental sound awareness, and at this point, the vast majority of newly implanted people terrifically exceed that, and they can chat on the phone, and participate fully in most conversations...so "reasonable expectations" have evolved over time! What is the current FDA status?
Greenberg: We have two FDA clinical trials underway...One is a long term clinical trial with 6 patients, and then we have a short term study underway too, and that one is evaluating the next generation device. We're hoping to do a multi-center trial in the next year or two. As of this moment, the Doheny Eye Institute at USC is the only center working with us, and several of the scientists and physicians that worked with me at Johns Hopkins were recruited by Doheny, and they are among the best retinal surgeons and scientists in this field.
Beck:Dr. Greenberg, my recollection was that the first visual implant was done in concert with three surgeons...an ENT surgeon, a retinal surgeon and an oculoplastic surgeon?
Greenberg: Yes, that's right it was a team of surgeons. But for the second generation, it'll just be the retinal surgeon and all of the electronics will be located within the eye itself.
Beck: Things are changing very quickly for visual implants and Second Sight. This is very exciting, thanks for allowing me to visit with you, and I am so grateful for your time.
Greenberg: Thanks Dr. Beck. It's wonderful to share our progress with you, and I'm grateful for your continued interest.
- - - - - - -
For More information about Second Sight, CLICK HERE.
CLICK HERE for more information on Advanced Bionics.
